Category Archives: False Claims
Warning: Medicare/caid Billing Confusion May Lead to Jail Time
All health care providers are under serious scrutiny, that is, if they take Medicaid. In Atlanta, GA, a dentist, Dr. Oluwatoyin Solarin was sentenced to a year and six months for filing false claims worth nearly $1 million. She pled guilty, and, I would assume, she had an attorney who recommended that she plead guilty. But were her claims actually false? Did she hire a criminal attorney or a Medicaid attorney? Because the answers could be the difference between being behind bars and freedom.
Dr. Solarin was accused of billing for and receiving payments for dental claims while she was not at the office. U.S. Attorney John Horn stated that “Solarin cheated the Medicaid program by submitting fraudulent claims, even billing the government for procedures she allegedly performed at the same time she was out of the country.”
I receive phone calls all the time from people who are under investigation for Medicare/caid fraud. What spurred on this particular blog was a phone call from (let’s call him) Dr. Jake, a dentist. He, similar to Dr. Solarin, was under investigation for Medicaid fraud by the federal government. By the time Dr. Jake called me, his investigation was well on its way, and his Medicaid reimbursements had been suspended due to credible allegations of fraud for almost a year. He was accused of billing for and receiving payments for dental services while he was on vacation…or sick…or otherwise indisposed. He hired one of the top criminal attorneys, who advised him to take a plea deal for a suspended jail sentence and monetary recompense.
But, wait, he says to me. I didn’t do anything wrong. Why should I have to admit to a felony charge and be punished for doing nothing wrong?
I said, let me guess, Jake. You were the rendering dentist – as in, your NPI number was on the billed claim – but you hired a temporary dentist to stand in your place while you were on vacation, sick, or otherwise indisposed?
How did you know? Jake asks.
Because I understand Medicaid billing.
When my car breaks down, I go to a mechanic, not a podiatrist. The same is true for health care providers undergoing investigation for Medicare/caid fraud – you need a Medicare/caid expert. A criminal attorney,most likely, will not understand the Medicare/caid policy on locum tenens. Or the legal limitations of Medicaid suspensions and the administrative route to get the suspension lifted. Or the good cause exception to suspensions.
Don’t get me wrong, I am not advocating that, when under criminal, health care fraud investigation, you should not hire a criminal attorney. Absolutely, you will want a criminal attorney. But you will also want a Medicare/caid attorney.
What is Locum tenens? It is a Latin phrase that means temporary substitute. Physicians and dentists hire locum tenens when they go on vacation or if they fall ill. It is similar to a substitute teacher. Some days I would love to hire a locum tenens for me. When a doctor or dentist hires a temporary substitute, usually that substitute is paid by the hour or by the services rendered. If the payor is Medicare or Medicaid, the substitute is not expected to submit the billing and wait to be reimbursed. The substitute is paid for the day(s) work, and the practice/physician/dentist bills Medicare/caid, which is reimbursed. For billing purposes, this could create a claim with the rendering NPI number as Dr. Jake, while Dr. Sub Sally actually rendered the service, because Dr. Jake was in the Bahamas. It would almost look like Dr. Jake were billing for services billing the government for procedures he allegedly performed at the same time he was out of the country.
Going back to Dr. Jake…had Dr. Jake hired a Medicare/caid attorney a year ago, when his suspension was first implemented, he may have be getting reimbursed by Medicaid this whole past year – just by asking for a good cause exception or by filing an injunction lifting the suspension. His Medicaid/care attorney could have enlightened the investigators on locum tenens, and, perhaps, the charges would have been dropped, once the billing was understood.
Going back to Dr. Solarin who pled guilty to accusations of billing for services while out of the country…what if it were just a locum tenens problem?
Medicare/Caid Audits: Urine Testing Under Fire!!
I have blogged about peeing in a cup before…but we will not be talking about dentists in this blog. Instead we will be discussing pain management physicians and peeing in a cup.
Pain management physicians are under intense scrutiny on the federal and state level due to increased urine testing. But is it the pain management doctors’ fault?
When I was little, my dad and I would play catch with bouncy balls. He would always play a dirty little trick, and I fell for it every time. He would toss one ball high in the air. While I was concentrating on catching that ball, he would hurl another ball straight at me, which, every time, smacked into me – leaving me disoriented as to what was happening. He would laugh and laugh. I was his Charlie Brown, and he was my Lucy. (Yes, I have done this to my child).
The point is that it is difficult to concentrate on more than one thing. When the Affordable Care Act (ACA) came out, it was as if the federal government wielded 500, metaphoric, bouncy balls at every health care provider. You couldn’t comprehend it in its entirety. There were different deadlines for multiple changes, provider requirements, employer requirements, consumer requirements…it was a bloodbath! [If you haven’t seen the brothers who trick their sister into thinking it’s a zombie apocalypse, you have to watch it!!]
A similar “metaphoric ball frenzy” is occurring now with urine testing, and pain management physicians make up the bulk of prescribed urine testing. The urine testing industry has boomed in the past 4-5 years. This could be caused by a number of factors:
- increase use of drugs (especially heroine and opioids),
- the tightening of regulations requiring physicians to monitor whether patients are abusing drugs,
- increase of pain management doctors purchasing mass-spectrometry machines and becoming their own lab,
- simply more people are complaining of pain, and
- the pharmaceutical industry’s direct-to-consumer advertising (DTCA).
Medicare’s spending on 22 high-tech tests for drugs of abuse hit $445 million in 2012, up 1,423% in five years. “In 2012, 259 million prescriptions were written for opioids, which is more than enough to give every American adult their own bottle of pills.” See article.
According to the American Association of Pain Management, pain affects more Americans than diabetes, heart disease and cancer combined. The chart below depicts the number of chronic pain sufferers compared to other major health conditions.
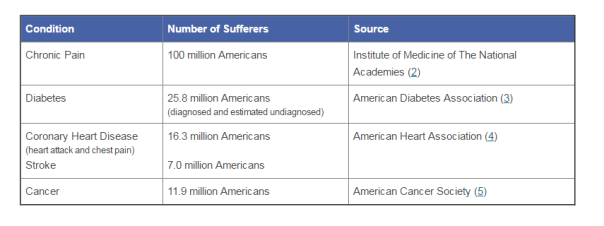
In the world of Medicare and Medicaid, where there is profit being made, the government comes a-knockin’.
But should we blame the pain management doctors if recent years brought more patients due to increase of drug use? The flip side is that we do not want doctors ordering urine tests unnecessarily. But aren’t the doctors supposed to the experts on medical necessity??? How can an auditor, who is not a physician and never seen the patient opine to medical necessity of a urine test?
The metaphoric ball frenzy:
There are so many investigations into urine testing going on right now.
Ball #1: The machine manufacturers. A couple of years ago, Carolina Liquid Chemistries (CLC) was raided by the federal government. See article. One of the allegations was that CLC was misrepresenting their product, a urinalysis machine, which caused doctors to overbill Medicare and Medicaid. According to a source, the federal government is still investigating CLC and all the physicians who purchased the urinalysis machine from CLC.
Ball #2: The federal government. Concurrently, the federal government is investigating urine testing billed to Medicare. In 2015, Millennium Health paid $256 million to resolve alleged violations of the False Claims Act for billing Medicare and Medicaid for medically unnecessary urine drug and genetic testing. I wonder if Millennium bought a urinalysis machine from CLC…
Ball #3: The state governments. Many state governments are investigating urine testing billed to Medicaid. Here are a few examples:
New Jersey: July 12, 2016, a couple and their diagnostic imaging companies were ordered to pay more than $7.75 million for knowingly submitting false claims to Medicare for thousands of falsified diagnostic test reports and the underlying tests.
Oklahoma: July 10, 2016, the Oklahoma attorney general’s office announced that it is investigating a group of laboratories involved in the state’s booming urine testing industry.
Tennessee: April 2016, two lab professionals from Bristol, Tenn., were convicted of health care fraud in a scheme involving urine tests for substance abuse treatments.
If you are a pain management physician, here are a few recommendations to, not necessarily avoid an audit (because that may be impossible), but recommendations on how to “win” an audit:
- Document, document, document. Explain why the urine test is medically necessary in your documents. An auditor is less likely to question something you wrote at the time of the testing, instead of well after the fact.
- Double check the CPT codes. These change often.
- Check your urinalysis machine. Who manufactured it? Is it performing accurately?
- Self-audit
- Have an experienced, knowledgeable, health care attorney. Do not wait for the results of the audit to contact an attorney.
And, perhaps, the most important – Do NOT just accept the results of an audit. Especially with allegations involving medical necessity…there are so many legal defenses built into regulations!! You turn around and throw a bouncy ball really high – and then…wallop them!!
Health Care Integration: A Glimpse Into My Crystal Ball
Throughout the history of health care, payors and payees of Medicare/caid have existed in separate silos. In fact, the two have combated – the relationship has not always been stellar.
Looking into my crystal ball; however, all will not be as it is now [that’s clear as mud!].
Now, and in the upcoming years, there will be a massive shift to integrate payors and payees under the same roof. Competition drives this movement. So does the uncertainty in the health care market. This means that under one umbrella may be the providers and the paying entities.
Why is this a concern? First – Any healthcare entity that submits claims to the federal government, whether it be a provider or payor, must comply with the fraud and abuse statutes. As such, there is a potential to run afoul of federal and state regulations regulating the business of health care. Payors know their rules; providers know their rules…And those rules are dissimilar; and, at times, conflicting. The opportunity to screw up is endemic.
Second – With the new responsibilities mandated by the Yates Memo, these new relationships could create awkward situations in which the head of the payor department could have knowledge (or should have knowledge) of an [alleged] overpayment, but because of the politics at the company or self-interest in the preservation of his or her career, the head may not want to disclose such overpayment. With the 60-day rule, the head’s hesitation could cost the company.
Let’s investigate:
The Affordable Care Act (ACA) reinvented health care in so many ways. Remember, the ACA is supposed to be self-funding. Taxes were not to increase due to its inception. Instead, health care providers fund the ACA through post payment and prepayment audits, ZPIC audits, CERTs, MFCU, MICs, RACs, and PERMs.
The ACA also made a whole new commercially-insured population subject to the False Claims Act. False statements are now being investigated in connection with Medical Loss Ratios, justifications for rate increases, risk corridor calculations, or risk adjustment submissions.
CMS imposes a duty to detect fraud, waste, and abuse (FWA). But what if you’re looking at your own partners?

The chart above depicts “old school” Medicare payment options for physicians and other health care providers. In our Brave New World, the arrows will be criss-crossed (applesauce), because when the payors and the payees merge, the reimbursements, the billing, and the regulatory supervision will be underneath the same roof. It’ll be the game of “chicken” taken to a whole new level…with prison and financial penalties for the loser.
Since 2011, kickback issues have exponentially grown. The Anti-Kickback Statute makes it a criminal offense for a provider to give “remuneration” to a physician in order to compensate the physician for past referrals or to induce future referrals of patients to the provider for items or services that are reimbursed, in whole or in part, by Medicare or Medicaid.
Imagine when payors and payees are owned by the same entity! Plus, the ACA amended the kickback statutes to eliminate the prong requiring actual knowledge or intent. Now you can be convicted of anti kickback issues without any actual knowledge it was ever occurring!!
Now we have the “one purpose test,” which holds that a payment or offer of remuneration violates the Anti-Kickback Statute so long as part of the purpose of a payment to a physician or other referral source by a provider or supplier is an inducement for past or future referrals. United States v. Borrasi, 2011 WL 1663373 (7th Cir. May 4, 2011).
There are statutory exceptions. But these exceptions differ depending on whether you are a payor or payee – see the potential criss-cross applesauce?
And, BTW, which types of health care services are bound by the anti kickback statutes?
- Clinical laboratory services;
- Physical therapy services;
- Occupation therapy services;
- Radiology services (including MRIs, Ultrasounds, and CAT scans);
- Radiation therapy and supplies;
- Durable medical equipment and supplies;
- Parenteral and enteral nutrients, equipment, and supplies;
- Prosthetics, orthotics, and prosthetic devices and supplies;
- Home health services;
- Outpatient prescription drugs; and
- Inpatient and outpatient hospital services.
Imagine a building. Inside is a primary care physician (PCP), a pediatrician, a home health agency, and a psychiatrist. Can the PCP refer to the home health agency? Can a hospital refer to a home care agency? What if one of the Board of Directors sit on both entities?
The keys to avoiding the anti kickback pitfalls is threefold: (1) fair market value (FMV); (2) arm’s length transactions; and (3) money cannot be germane to referrals.
However, there is no one acceptable way to determine FMV. Hire an objective appraiser. While hiring an objective appraiser does not establish accuracy, it can demonstrate a good faith attempt.
Number One Rule for Merging/Acquiring/Creating New Partnerships in our new Brave New World of health care?
Your attorney should be your new BFF!! (Unless she already is).
CMS Releases Final Rule On Return of Overpayments
Written by: David Leatherberry, partner in Gordon &Rees‘ San Diego office
The Centers for Medicare & Medicaid Services released its final rule today on the return of overpayments. The final rule requires providers and suppliers receiving funds under the Medicare/Medicaid program to report and return overpayments within 60 days of identifying the overpayment, or the date a corresponding cost report is due, whichever is later. As published in the February 12, 2016 Federal Register, the final rule clarifies the meaning of overpayment identification, the required lookback period, and the methods available for reporting and returning identified overpayments to CMS. See https://www.federalregister.gov/articles/2016/02/12/2016-02789/medicare-program-reporting-and-returning-of-overpayments.
Identification
The point in time in which an overpayment is identified is significant because it triggers the start of the 60-day period in which overpayments must be returned. CMS originally proposed that an overpayment is identified only when “the person has actual knowledge of the existence of the overpayment or acts in reckless disregard or deliberate ignorance of the overpayment.” The final rule changes the meaning of identification, stating that “a person has identified an overpayment when the person has or should have, through the exercise of reasonable diligence, determined that the person has received an overpayment and quantified the amount of the overpayment. The change places a burden on healthcare providers and suppliers to have reasonable policies and programs in place which monitor the receipt of Medicare/Medicaid payments.
6-Year Lookback Period
The final rule also softens the period for which health care providers and suppliers may be liable for the return of overpayments. As the rule was originally proposed, CMS required a 10-year lookback period, consistent with the False Claims Act. Now, overpayments must be reported and returned only if a person identifies the overpayment within six years of the date the overpayment was received.
Guidance in Reporting and Returning Overpayments
The final rule provides that providers and suppliers must use an applicable claims adjustment, credit balance, self-reported refund, or other appropriate process to satisfy the obligation to report and return overpayments. If a health care provider or supplier has reported a self-identified overpayment to either the Self-Referral Disclosure Protocol managed by CMS or the Self-Disclosure Protocol managed by the Office of the Inspector General (OIG), the provider or supplier is considered to be in compliance with the provisions of this rule as long as they are actively engaged in the respective protocol.
The False Claims Act: Are You Just Shaking a Magic 8 Ball?
Often we read in the news stories of hospitals or health care providers paying inordinate amounts to settle cases in which credible allegations of fraud or allegations of false claims preside. Many times the providers actually committed fraud, waste, or abuse. Maybe medical records were falsified, or maybe the documents were created for Medicaid/care recipients that do not exist. Maybe the services claimed to have been rendered were not. In these cases, the provider can be held liable criminally (fraud) and/or civilly (false claims). And these providers should be held accountable to the government and the taxpayers.
It appears that this is not the case for an Ohio hospital that settled a False Claims Act case for $4.1 million last month. Do not get me wrong: The False Claims Act is no joke. Possible penalties imposed by the False Claims Act can be up to $10,000 per claim “plus 3 times the amount of damages which the Government sustains because of the act of that person.” 37 USC §3729. See blog for more explanation.
In the Ohio hospital’s case, the penalty derived from Dr. Abubakar Atiq Durrani, a spinal surgeon, performing spinal surgeries that, allegedly, were not medically necessary.
According to what I’ve read, there is no question that Dr. Durrani actually performed these surgeries. He did. On actual people who exist. Instead, the allegation is that the surgeries were not medically necessary.
I have blogged about medical necessity in the past. Medical necessity is a subjective standard. Medical necessity is defined as reasonable, necessary, and/or appropriate, based on evidence-based clinical standards of care.
But it is still a subjective standard. When you receive news that you suffer from a debilitating disease, what do you do? You get a second opinion. If one doctor recommends brain surgery, what do you do? You get a second opinion.
After that, you grab a handy, dandy Magic 8 Ball and give it a shake. Kidding. Kinda.

My point is that 2 physicians can recommend two different courses of treatment. One physician may practice more defensive medicine, while another may be more cautious. Surgeons will, generally, recommend surgery, more than non-surgeons; it’s what they do.
Going back to Dr. Durrani, who was arrested in 2013 for allegedly “convinc[ing] [patients] they needed spine and neck surgery. However, other doctors later determined those surgeries as unnecessary and damaging to the patient’s health.”
I find two points striking about this case: (1) The allegation that this physician “convinced” people to undergo spine surgery; and (2) The fact that the hospital settled for $4.1 million when no fraud existed or was alleged, only questions as to medical necessity, which is subjective.
As to the first, I am imagining my doctor. I am imagining that I have horrible, chronic back pain. My doctor recommends spinal surgery. There is no way, at all, ever, in this universe, that any doctor would be able to convince me to undergo surgery if I did not want surgery. Period. Who allows themselves to be peer pressured into surgery? Not to knock on my own profession, but I have a sneaky suspicion that this allegation was concocted by the plaintiffs’ attorney(s) and the plaintiffs responded, “Oh, you are right. I was persuaded.”
As to the second…Why did the hospital settle for such a high amount? Couldn’t the hospital have gone to trial and convinced a jury that Dr. Durrani’s surgeries were, in fact, reasonable and/or appropriate, based on evidence-based clinical standards of care?
According to the Magic 8 Ball, “signs point to yes.” Why cave at such a large number where no fraud was alleged?
Whatever happened to Dr. Durrani because of this whole mess? “Following his arraignment, Durrani allegedly fled the United States and remains a fugitive.”
In sum, based on allegations of questionable medical necessity, not fraud, a hospital paid $4.1 million and a U.S. physician fled into hiding…allegedly.
I question this outcome. I even question whether these types of allegations fall within the False Claims Act.
The False Claims Act holds providers liable for (abridged version):
- knowingly presenting a fraudulent claim to the Government;
- knowingly making a fraudulent record or statement to the Government;
- conspiring to do any of the referenced bullet points;
- having possession of Government money and knowingly delivering less than the amount;
- delivering a certified document intending to defraud the Government without completely knowing whether the information was true;
- knowingly buying or receiving as a pledge of debt, public property from the an employee of the Government who does not have the right to pledge that property;
- knowingly making, using, or causing to be made or used, a false record material to an obligation to pay the Government, or knowingly concealing or decreasing an obligation to pay the Government.
I see nothing in the False Claims Act punishing a provider for rendering services that, perhaps, may not be medically necessary.
I actually find questions of medical necessity to be easily defensible. After all, who do we look to for determinations of what are reasonable and/or appropriate services, based on evidence-based clinical standards of care?
Our physicians.
Sure, some physicians may have conflicting views as to what is medically necessary. I see it all the time in court. One expert witness physician testifies that the service was medically necessary and another, equally as qualified, physician testifies to the contrary.
Unless I’m missing something (here, folks, is my “CYA”), I just do not understand why allegations of questionable medical necessity caused an U.S. physician to become a fugitive and a hospital to settle for $4.1 million.
It’s as if the hospital shook the Magic 8 Ball and asked whether it would be able to defend itself and received:
Tightrope Walking: Correcting Errors in Health Care Documents After the Fact
People screw up. We are human; hence the term, “human error.”
But how to handle said mistakes in health care records after the fact, which could be the target in a Medicare/caid audit?
This is a very important, yet extremely “fine-lined” topic. Imagine a tightrope walker. If you fall off one way, you fall to the abyss of accusations of fraud. You fall off the other way and you fall into the ocean of the False Claims Act. Fixing document errors post date of service (DOS) is a fine line with catastrophic consequences on both sides.
In NC, our administrative code provides guidance.
“SECTION .1400 – SERVICE RECORDS
10A NCAC 13J .1401 REQUIREMENT
(a) The agency shall develop and implement written policies governing content and handling of client records.
(b) The agency shall maintain a client record for each client. Each page of the client record shall have the client’s name. All entries in the record shall reflect the actual date of entry. When agency staff make additional, late, or out of sequence entries into the client record, the documentation shall include the following applicable notations: addendum, late entry, or entry out of sequence, and the date of the entry. A system for maintaining originals and copies shall be described in the agency policies and procedures.
(c) The agency shall assure that originals of client records are kept confidential and secure on the licensed premises unless in accordance with Rule .0905 of this Subchapter, or subpoenaed by a court of legal jurisdiction, or to conduct an evaluation as required in Rule .1004 of this Subchapter.
(d) If a record is removed to conduct an evaluation, the record shall be returned to the agency premises within five working days. The agency shall maintain a sign out log that includes to whom the record was released, client’s name and date removed. Only authorized staff or other persons authorized by law may remove the record for these purposes.
(e) A copy of the client record for each client must be readily available to the appropriate health professional(s) providing services or managing the delivery of such services.
(f) Client records shall be retained for a period of not less than five years from the date of the most recent discharge of the client, unless the client is a minor in which case the record must be retained until three years after the client’s 18th birthday. When an agency ceases operation, the Department shall be notified in writing where the records will be stored for the required retention period.”
What NOT to do:
- Erase notations and write the revision
- Add a check mark that was not previously there
- Forge a staff’s initials
- Back date the revision
When it comes to alteration of medical records for Medicare/caid patients after the DOS, you are walking on a tightrope. Catastrophe is below, not a net. So tiptoe carefully.
Call an attorney with specific questions.
False Claims Act: The Medicare Horror Story
What the heck is the False Claims Act and why is it important to you?
When it comes to Medicaid and Medicare, the ghoulish phrase “False Claims Act” is frequently thrown around. If you google False Claims Act (FCA) under the “news” option, you will see some chilling news article titles.
- Pediatric Services of America, units to pay $6.88 in False Claims
- NuVasive, Inc. Agrees to Pay $13.5 Million to Resolve False Claims
- California Oncologist Pays $736k to Settle False Claims Allegations
False claims cases tend to be high dollar cases for health care providers; many times the amounts are at issue that could potentially put the provider out of business. FCA is spine-chilling, and many health care providers would rather play the hiding child rather than the curious investigator in a horror story. Come on, let’s face it, the curious characters usually get killed. But, this is not a horror story, and it is imperative that providers are informed of the FCA and potential penalties.
I have blogged about post payment reviews that use extrapolation, which result in astronomical alleged overpayments. See blog and blog. Interestingly, these alleged overpayments could also be false claims. It is just a matter of which governmental agency is pursuing it (or person in the case of qui tem cases).
But the ramifications of false claims allegations are even more bloodcurdling than the astronomical alleged overpayments. It is important for you to understand what false claims are and how to prevent yourself from ever participating in a false claim, knowingly or unknowingly.
First, what is a false claim?
A false claims occurs when you knowingly present, or cause to be presented, to the US Government a false or fraudulent claim for payment or approval. (abridged version).
Let’s analyze.
The false claim does not have to be billed with actual knowledge that it is false or fraudulent. The false claim does not even have to be fraudulent; it can be merely false. The distinction lies in that a fraudulent claim is one that you intentionally alter. A false claim could merely be incorrect information. Saying it another way, the false claim can be a false or incorrect claim that you had no actual knowledge was false. That is hair-raising.
What is the penalty? It is:
A civil penalty of not less than $5,500 and not more than $11,000 per claim, plus 3 times the amount of the claim. You can see why these are high dollar cases.
The federal government recovered a jaw-dropping $5.7 billion in 2014 under the False Claims Act (FCA). In 2013, the feds recovered $5 billion under the FCA. Expect 2015 to be even higher. Since the inception of the Affordable Care Act (ACA), FCA investigations have increased.
Overwhelmingly, the recoveries are from the health care industry.
Everyone knows that the Medicare Claims Processing Manual is esoteric, verbose, and vague. Let’s face it: just Chapter 1 “General Billing Requirements” alone is 313 pages! Besides me, who reads the Medicare Claims Processing Manual cover to cover? Who, besides me, needs to know that Medicare does not cover deported beneficiaries or the exceptions to the Anti-markup Payment Limitation?
Not to mention, the Manual is not law. The Manual does not get approved by Congress. The Manual is guidance or policy.
However, in FCA cases, you can be held liable for items in the Medicare Claims Processing Manual of which you were not aware. In other words, in FCA cases, you can be found liable for what you should have known.
Real life hypotheticals:
Hospital submits claims to Medicare and received payment for services rendered in a clinical trial involving devices to improve organ transplants. Unbeknownst to the hospital, the Manual prohibits Medicare reimbursements for non-FDA approved services.
Physician A has reciprocal arrangement with Physician B. A undergoes personal surgery and B serves A’s Medicare Part B patients while A is recovering. A returns and bills Medicare and is paid for services rendered by B 61 days+ after A left the office.
A physician accepts assignment of a bill of $300 for covered Medicare services and collects $80 from the enrollee. Physician neglects to depict on the claim form that he/she collected anything from the patient. Medicare’s allowable amount is $250, and since the deductible had previously been met, makes payment of $200 to the physician.
These are just a few examples of situations which could result in a FCA allegation.
But do not fret! There are legal defenses written into the Social Security Act that provides protection for health care providers!
Important take-aways:
1. Check whether you have insurance coverage for FCA.
2. Have an attorney on hand with FCA experience.
3. Read portions of the Medicare Claims Billing Manual which are pertinent to you.
Most importantly, if you are accused of billing false claims, get your advocate sooner rather than later! Do not engage in any conversations or interviews without counsel!
Appeal all findings!
Knicole Emanuel Speaks About CSC Fraud Investigation on ABC
I was interviewed by Heather Waliga, ABC News, last Friday about the U.S. Attorney’s lawsuit against Computer Sciences Corporation (CSC) accusing CSC of hundreds of millions of dollars of Medicaid fraud.
To watch the video, please click here.
But, beware! Do not make the video full screen unless you are prepared to see a very, large, close-up picture of my head. The camera man zoomed in to, literally, just my head.
NCTracks developer CSC faces health care fraud lawsuit in NY
Jason DeBruyn of the Triangle Business Journal wrote:
Computer Sciences Corporation, the company that designed, developed and is operating the Medicaid claims payment system in North Carolina, is facing a health care fraud lawsuit brought by the U.S. attorney’s office in New York.
That lawsuit has no immediate impact in North Carolina, though Computer Sciences Corp. (CSC) built the system in this state – called NCTracks – using 32 percent of the code used in New York City. Initially, CSC had hoped to duplicate as much as 73 percent of the New York City code in North Carolina.
NCTracks has been the target of several attacks from health care providers who say they have not been paid on time. The N.C. Department of Health and Human Services, where NCTracks is housed, faces a lawsuit that could incorporate 70,000 health care providers and end up with damages exceeding $100 million. NCTracks has been the target of at least three searing audits.
The New York lawsuit, brought by Preet Bharara, the U.S. Attorney for the Southern District of New York, alleges billing fraud schemes that used computer programs to automatically alter billing data, including the use of a defaulting program to systematically falsify diagnosis codes submitted to Medicaid.
“As alleged, CSC and the City created computer programs that systematically, and fraudulently, altered billing data in order to get paid by Medicaid as quickly as possible and as much as possible,” Bharara said through a statement. “Billing frauds like those alleged undermine the integrity of public healthcare programs like Medicaid.”
Although this lawsuit makes no mention of activity in North Carolina, Knicole Emanuel, an attorney with Williams Mullen in Raleigh who represents providers in the lawsuit against DHHS, says it “will almost certainly cause the federal government to peer a bit closer at all CSC’s billing software systems in other states (including North Carolina).”
Representatives from DHHS did not immediately comment on the New York lawsuit.






